
March 29, 2020
In these challenging times, we hope the following COVID-19 update will have value as you face challenges related to the safe use of medicines for COVID-19. It is being sent to all healthcare providers and/or research scientists registered to receive information from the CredibleMeds website. Today, there are many drugs known to cause torsades de pointes (TdP) that are either being tested in clinical trials or used off-label, sometimes with little or no medical supervision.
Here are a few key points we recommend be considered:
- At this time, no drug therapy has been proven to be effective for COVID-19. The available studies are small and either negative or seriously flawed.
- Several antivirals (including favipiravir, remdesivir, umifenovir and lopinavir/ritonavir) and anti-inflammatory antibiotic drugs (azithromycin) and antimalarials (chloroquine and hydroxychloroquine) are now being tested alone and in combination for their potential efficacy.
- Chloroquine, hydroxychloroquine and azithromycin are all on the Known Risk of TdP list.
- The combination lopinavir/ritonavir is known to cause QT prolongation and is on the Possible Risk of TdP list. The drug label for lopinavir/ritonavir includes a strong warning to "avoid use with QT-prolonging drugs" because of drug-interactions and the drugs' effects on PR and QTc.
- Single oral doses of Favipiravir (1200 and 2400 mg) did not prolong QTc in a study of 56 normal subjects. (PMID 26308176 ) but we have not found evidence that remdesivir or umifenovir have been tested for their effects on QTc. We have not found reports of QT prolongation or TdP for these drugs in FAERS or WHO’s Vigibase. (We use Oracle’s Empirica Signal datamining software).
- The very ill patients being treated with these drugs are also those who are most likely to have one or more of the clinical conditions known to prolong QTc (https://crediblemeds.org/index.php?cID=381) and thereby add to their TdP risk (heart disease, bradycardia, hypokalemia, hypoxia, etc.).
- These very ill patients are also likely to receive concomitant drugs that can prolong QTc and increase risk of TdP, e.g., antiemetics such as ondansetron, PPIs for acid suppression and anesthetic agents.
Recommendations:
- If drug therapy is justified and considered necessary, we encourage all to offer these patients participation in a clinical trial, if one is available so that we can gain better evidence for the safety and effectiveness of these drugs.
- We do not recommend off-label use of these drugs but, if a trial is not available or is declined, the known risks and the absence of evidence for efficacy should be explained to the patient.
- I reiterate, we do not recommend off-label use but, if that is the only option for very ill patients, select a therapy that has, based on the least likelihood of harm and some scientific basis for its choice.
- Also, do not begin with a drug combination (as reported in the press) until a single agent has been tried.
- If drugs with QTc risk are to be administered, we advise all to follow the recommendations just released by Dr. Michael Ackerman and his colleagues at Mayo Clinic. The flow diagram below is taken from his article in Mayo Clinic Proceedings (online) and gives suggestions for the management of QT risk in these clinical situations.
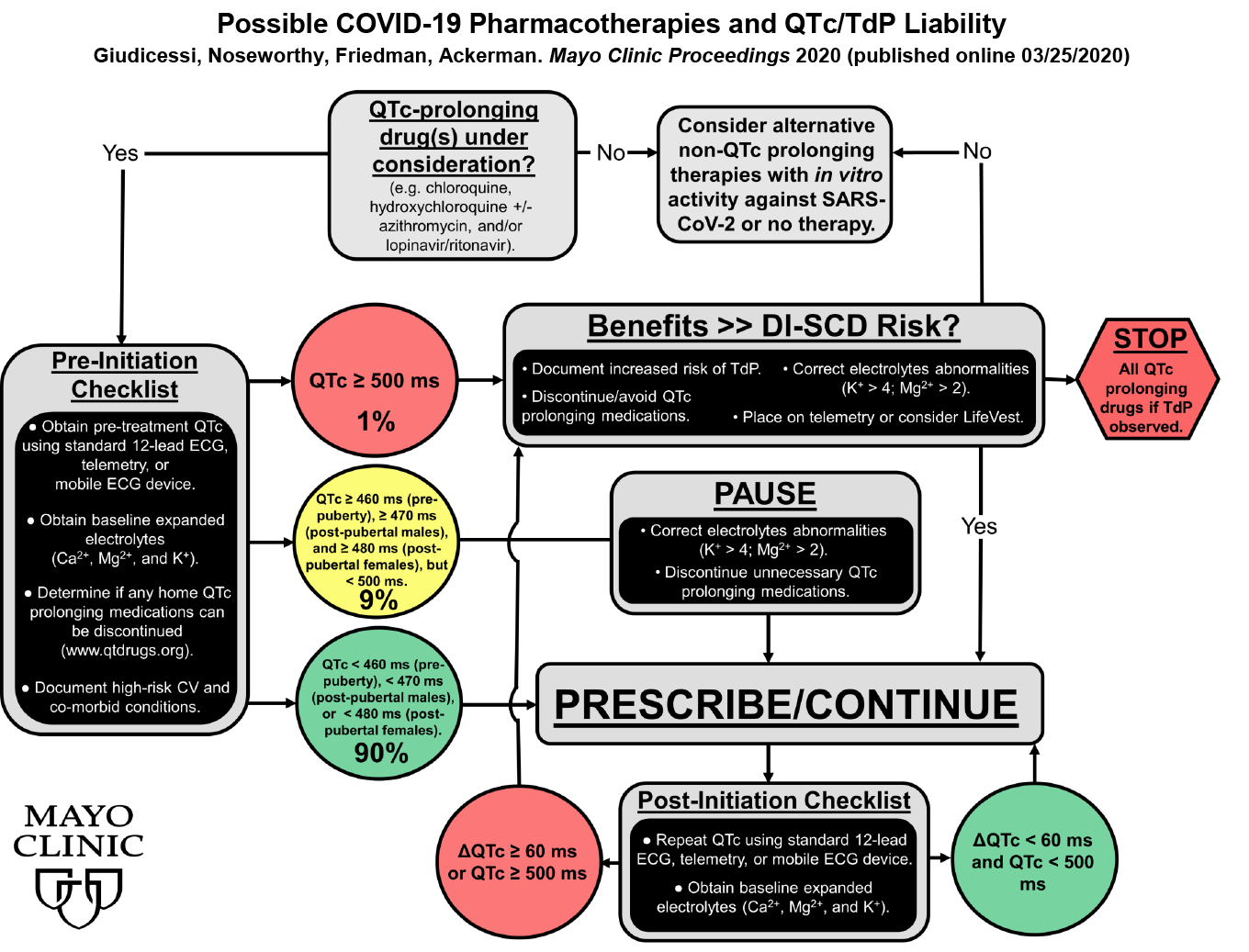
- We hope you will follow his advice and use the CredibleMeds lists of drugs to screen and assess risk before any new QT-prolonging drug is prescribed. Likewise, please use QTdrugs lists to monitor any subsequent changes in therapy. Remember that the list is also available in a free smartphone app (link on the CredibleMeds homepage).
- Lastly, if available in your EMR, we strongly encourage the use of decision support systems to identify patients at high risk of TdP by monitoring the patients’ risk factors and medications. These systems automatically check the CredibleMeds QTdrugs list, generate QT Alerts and offer guidance for risk management. They are now being used in many institutions such as Mayo Clinic, Indiana U., U. Colorado, Belgium, Netherlands and Banner Health in the US Southwest, the latter, made possible by an award from the FDA to AZCERT.
Thank you for your support of CredibleMeds and your interest in QTdrugs.org. We always value your feedback for how CredibleMeds can better serve you and your safe use of medicines.
Raymond L. Woosley, MD, PhD
![]()

